Eye drop recall
A majority of those affected reported using preservative-free EzriCare Artificial Tears before they became. Two other products.

Eye Drops Sold Worldwide Recalled Due To Non Sterility Cnet
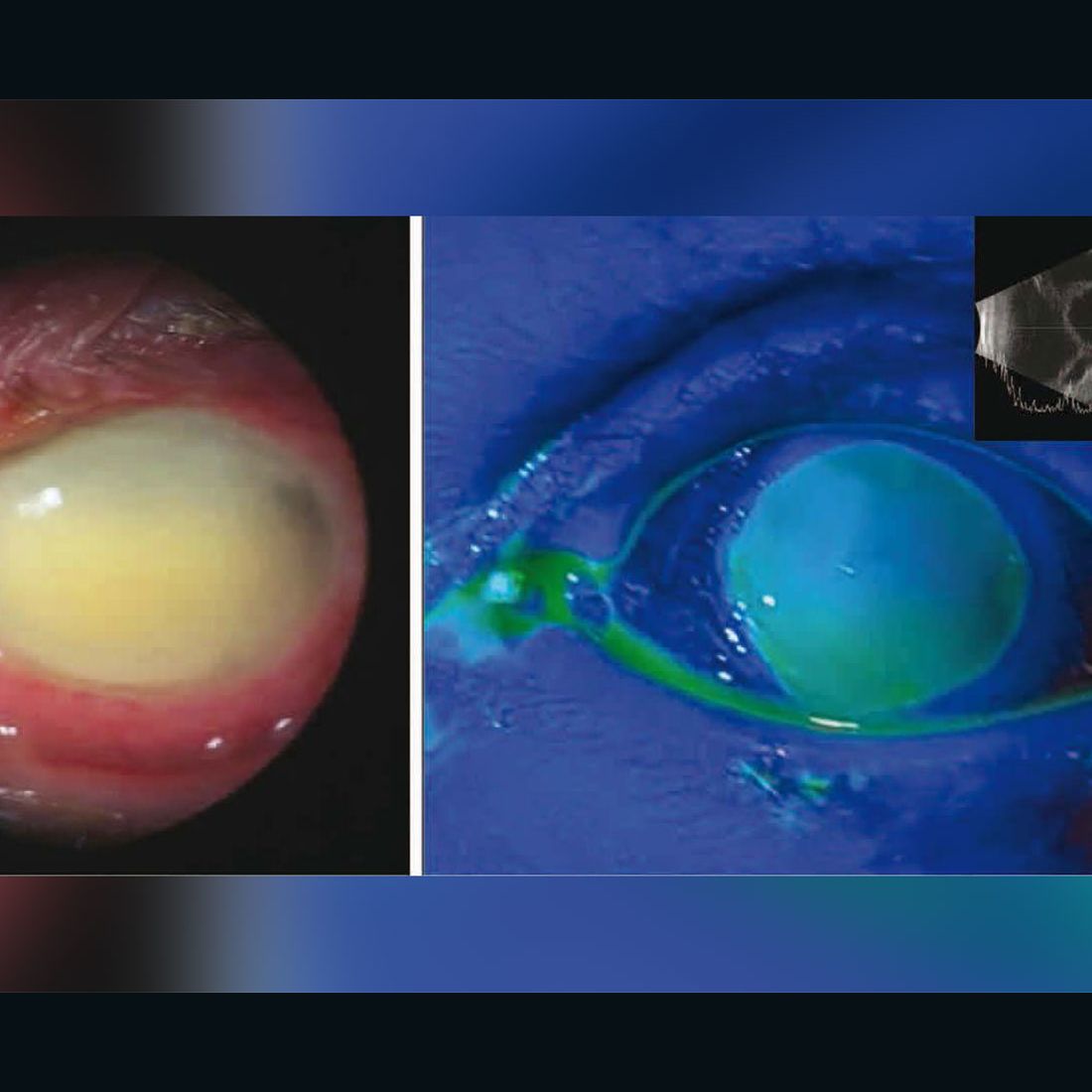
Web Eye drop manufacturer issues recall amid CDC investigation of infections death The mans vision at his two months follow-up appointment was 20400 meaning he can see at 20 feet what healthy.

. The two lots were pulled because of problems that could result in blindness the company said. Web Two types of artificial tears eye drops were voluntarily recalled after 55 reports of eye infections vision loss and even a bloodstream infection that led to one death according to officials. Web The Phoenix-based company said consumers should immediately stop using the drops and return them to the place they were purchased.
Web 1 day agoManufacturer recalls eyedrops after possible link to bacterial infections. Web 5 hours agoAt least four eyedrops have been recalled recently. Consumers are advised to stop using the following brands and return them to the place of purchase.
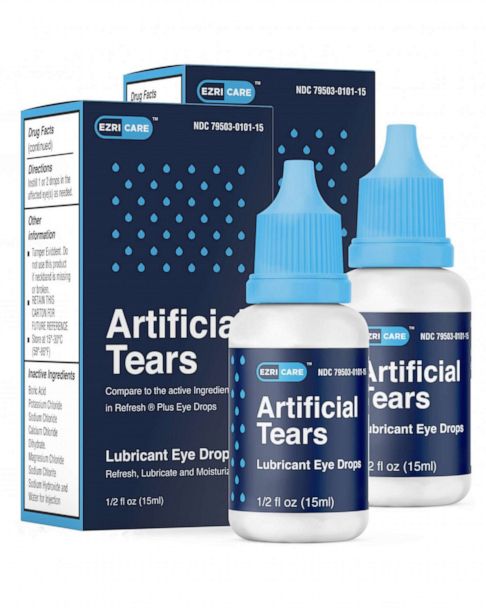
They include EzriCare and Delsam Pharmas Artificial Tears which have been associated with the bacterial infection. Web On Thursday the maker of the eyedrops recalled them because of possible contamination. UC Davis Health experts share what you need to know.
Web The Food and Drug Administration FDA has recently recalled three brands of eye drops including one that has been linked to serious infections vision loss and a death. Web Two types of artificial tears eye drops have been voluntarily recalled following 55 reports of adverse use effects including eye infections vision loss and even a bloodstream infection that led. Per the CDCs latest update 68 patients across 16 states have been infected with Pseudomonas aeruginosa according to.
Web CNNGlobal Pharma Healthcare is issuing a recall of its Artificial Tears Lubricant Eye Drops that were distributed by EzriCare and Delsam Pharma due to possible contamination the US Food and. Web Pharmedica is recalling its Purely Soothing 15 MSM Drops meant to treat eye irritation. The recall affects nearly 2900 bottles according to the company.
Eye Drops Recalled After 55 Reports Of Bacterial Infection 1 Death In 12 States Good Morning America

Wmjzquw2m5l8dm
Eye Drop Recall Announced Due To Possible Bacteria Contamination Top Class Actions

Eye Drop Product Recall The Portugal News

Cdc Sounds Alarm On Eye Drops Linked With Drug Resistant Infection Medpage Today

27jcbcjyde0vam
O N2vpc3liz1vm
:quality(70)/cloudfront-us-east-1.images.arcpublishing.com/cmg/7RXMEOM35FFEFBAMLZK7HWIWUM.jpg)
Recall Alert Fda Announces Two More Eyedrop Recalls Due To Potential Contamination Kiro 7 News Seattle

Rohto Eye Drops Recall Due To Sterility Concerns Aboutlawsuits Com

2 More Eye Drop Brands Recalled Due To Safety Risks

Eye Drops Are Recalled After Being Linked To Vision Loss And 1 Death The New York Times

Contamination Risks That Could Cause Injuries Cited In Recalls Of Two Eye Drop Brands Fox31 Denver

Fda Recalls 3 Brands Of Eye Drops What Patients Need To Know

Eye Drop Recall Everything You Need To Know Right Now

Ezricare Eye Drops Recall Linked To Infections Blindness Death
9qh5htchxgadam

Eyedrop Recall Two More People Die Amid Outbreak Of Bacteria Fox Business